Abstract
Background: Mortality in pediatric acute myeloid leukemia (AML) has plateaued between 30-40%, with 5-15% of these deaths occurring during initial induction. While pulmonary complications contribute to the early morbidity and mortality in pediatric AML, our knowledge of the incidence, risk factors, and patterns of respiratory adverse events (AEs) is lacking. Hypothesized risk factors for respiratory AEs during induction include hyperleukocytosis, fluid overload, coagulopathy, and infection. Our objective was to characterize the incidence, categories, and risk factors of respiratory AEs in de novo pediatric AML patients during induction chemotherapy.
Methods: We conducted a single institution retrospective longitudinal study from presentation to Day 42 or initiation of the next chemotherapy cycle in de novo pediatric AML patients (age 0-21 years) presenting between January 1, 2001 and December 31, 2016. Patients were identified through the institutional cancer registry. Exclusion criteria included: relapsed AML, induction at another institution, and failure to treat with conventional protocols. Outcomes of interest included development of an NCI CTCAE (v4.0) grade 2-5 respiratory AE or death from another cause. Data was abstracted in regards to demographic and disease characteristics at presentation. Supportive care measures included fluid status, with fluid overload defined as a ≥ 10% increase from body weight at presentation or the use of ≥ 3 doses of furosemide during the follow up period. Respiratory AE was attributed to infection in cases with a positive bacterial or fungal blood culture, respiratory viral panel, endotracheal tube aspirate, or a chest imaging report explicitly suggesting a pulmonary infectious process. A single rater assigned the most specific, best fitting CTCAE category and grade for each incident event at its maximal severity. Time to event distribution curves were created and descriptive statistics using two-sample T-test, Chi square test for independence, or Fisher's exact test as appropriate were calculated (SAS v X, Cary, NC).
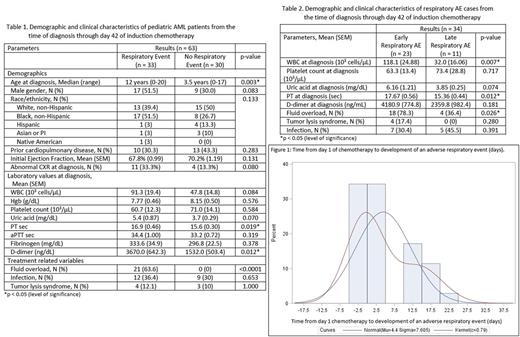
Results: To date we have reviewed the induction course on 63 of 245 eligible patients with de novo AML. Median age at diagnosis was 8.0 years (range, 0-21 years), 58.7% were female (n = 37), and 42.9% non-Hispanic White (n = 28). During the follow up period, 3 patients died (4.8% induction death at days 5, 25, 73). The deaths at days 25 and 73 were due to pulmonary fungal infection and the death at day 5 from an intracranial bleed. During the first 42 days, 52.4% (n = 33) patients experienced 34 discrete respiratory AEs: CTCAE grade 2 in 2.9% (n = 1), grade 3 in 64.7% (n = 22), grade 4 in 26.5% (n = 9) and grade 5 in 5.9% (n = 2). Among the respiratory AEs, 44.1% required BiPAP/CPAP (n = 4) or mechanical ventilation (n = 11). As shown in Table 1, those with and without respiratory AEs differed significantly in regards to median age at diagnosis (p = .003), coagulopathy at diagnosis (PT (p = .019), D-dimer (p = .012)), and fluid overload status (p < .0001). Those with respiratory AEs did not differ by sex (p = .08), development of infection (p = .65), or WBC at diagnosis (p = .08). A bimodal distribution of time to event was (Figure 1) with 67.7% (n = 23) of AEs occurring between presentation and day 7 and classified as "early." As seen in Table 2, there were statistically significant differences between the early and late AE groups for WBC at diagnosis (p = .007), PT at diagnosis (p = .012), and fluid overload (p = .026). While a larger proportion of the late AEs experienced infection (45.5% vs 30.4%), this did not significantly differ from the early AEs (p = .39).
Conclusion: We note a high incidence of grade 2-5 respiratory AEs in pediatric patients during AML initial induction [52.4%]. Fluid overload appears to be a significant factor in early AEs. Documented infection occurred in a higher proportion of late AE cases, although this did not reach statistical significance. Future analyses will examine the role of cytogenetic factors and morphology. Pulmonology and oncology raters will independently characterize and validate the category of each respiratory AE (e.g. atelectasis, pulmonary edema, pulmonary leukostasis, pneumonia) through a standardized process for the full eligible cohort. Identifying modifiable risk factors will enable design of supportive care interventions to decrease the risk of respiratory AEs and their associated morbidities during induction.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal